What Are Natural Zeolites
Natural zeolites are the centerpiece of BioGreen’s efforts to benefit CAFOs. We recommend their application as a feed additive, for animal bedding, and their incorporation with animal manures to produce a nitrogen-rich, high-quality organic fertilizer for field crops. In order to appreciate what natural zeolites can do for CAFOs and their surrounding environment, it is useful to understand what they are and how they work.
First discovered 1756, natural zeolites are hydrated sodium, potassium, and calcium aluminosilicate minerals found in volcanogenic sedimentary rocks worldwide. Formed by the gradual modification of volcanic ash deposited in lake and marine waters eons ago, large deposits of natural zeolites were first discovered in the western US, Mexico, Eastern Europe and Asia beginning in the 1950s. This was also about the time synthetic zeolites were being developed as “molecular sieves” for various industrial uses.
There are over 80 different natural zeolites, but only a few common types represent the bulk of the available natural zeolites worldwide. Clinoptilolite (Na3K3)6(Al12Si30O72).24H2O, together with heulandite, chabazite, modenite, and philipsite, are the most common types of natural zeolites.
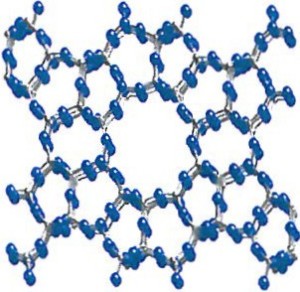
Clinoptilolite, like many zeolites, is characterized by a crystalline structure with open three-dimensional channels throughout (illustrated on left). This lattice, “honeycomb” structure makes zeolite relatively lightweight, soft, and porous. This unique physical structure – think of a rigid dried-out sponge – is also critical in zeolite’s important properties, including cation exchange and dehydration-rehydration.
Properties
Natural zeolites possess several important properties including cation exchange, dehydration-rehydration, and adsorption. Considerable scientific research in the last few decades has identified broad applications (see Scientific Support) for natural zeolites that takes advantage of these unique properties. For optimizing large-scale CAFO animal feed and manure management as well as agricultural applications, cation exchange and dehydration-rehydration are zeolite’s two most important properties to consider.
Cation exchange: Cations are positively charged ions. For example, aluminum (Al3+), calcium (Ca2+), sodium (Na+) and potassium (K+) are all cations found in natural zeolites. The ammonium ion (NH4+) is also a cation when in an aqueous solution. Cation exchange occurs when weakly bonded cations are removed or exchanged with a strong solution of another cation. A substance’s potential for this exchange is called the “cation exchange capacity”, or CEC. Natural zeolites have CECs from about 2-4 milliequivalents per gram (meq/g), which is twice the CEC of bentonite clay.
Clinoptilolite zeolite has an especially strong affinity for NH4+, exchanging with K+ and Na+ ions within the  clinoptilolite’s honeycomb structure. This ammonia can remain sequestered in the zeolite for long-term storage for two primary reasons. First, the ammonium ions bound within the zeolite are safe from volatilization, minimizing nitrogen losses to the atmosphere and associated noxious odors. Second, the pore openings within zeolite’s honeycomb structure are too small (4-5 angstroms) for nitrifying bacteria to enter. Therefore, ammonia sequestered within zeolite cannot be oxidized to nitrate – and subsequently be lost from the farm through nitrate-rich agricultural runoff that pollutes downstream waterways and basins.
clinoptilolite’s honeycomb structure. This ammonia can remain sequestered in the zeolite for long-term storage for two primary reasons. First, the ammonium ions bound within the zeolite are safe from volatilization, minimizing nitrogen losses to the atmosphere and associated noxious odors. Second, the pore openings within zeolite’s honeycomb structure are too small (4-5 angstroms) for nitrifying bacteria to enter. Therefore, ammonia sequestered within zeolite cannot be oxidized to nitrate – and subsequently be lost from the farm through nitrate-rich agricultural runoff that pollutes downstream waterways and basins.
Dehydration-rehydration: When zeolite is put into water, bubbles may appear as water fills in the voids of the open lattice, honeycomb structure. This bubble formation is responsible for the name “zeolite,” which means “boiling stone” in Greek. Contrary to synthetically made polymers and clay (for example, bentonite) minerals, clinoptilolite adsorbs and releases up to 70% by weight in water without expansion of its volume. This property makes natural zeolites exceptionally valuable for agricultural soils, particularly in semi-arid regions where water is costly and efficient water conservation is critical. When used in animal feeds, the zeolites consumed make the resulting manure less liquid, less odiferous, and easier to handle.

